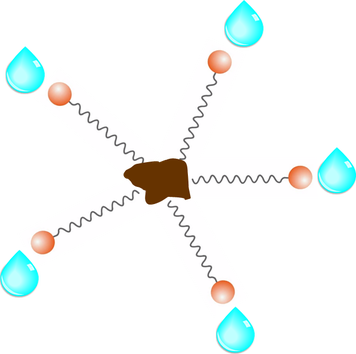
 Soap...it's something we all have used a zillion times over in our lifetime...but do you know how it really works? Let's take a look at how soap helps water wash away soil and dirt from our skin, clothes, dishes, and anything else we want to clean... Chemistry 101 Here's an experiment for those of you who'd like to revisit your high school chemistry class days......come on, you know you want to try this! 1- Take a small jar and add about 1/2 jar of water and a couple tablespoons of oil. Place the cap on and shake. After a few minutes, what do you notice? The water and oil should have separated with the oil laying on top of the water...right? 2- Now add several drops of dish-washing liquid and shake. Let sit a few minutes. Now what do you see? The mixture should be cloudy since the oil and water have mixed together because of the added soap. They aren't separating like before. Why not? Soap MoleculesSoap molecules have long hydrocarbon chains or "tails" with a carboxylate "head". The soap molecule's tail is hydrophobic which means it hates water but it is attracted to soil, oil, and dirt. The soap molecule's "head" is hydrophilic which means it loves water and is attracted to water molecules. With this opposite attraction, the soap molecule is the binding force that holds water and oil or dirt together. As the water is rinsed away, the oil and dirt rinses away with it. Simple, huh? Who said Chemistry class wasn't fun!!??
Comments are closed.
|
Welcome to Tailored Tidbits!If I'm not in the kitchen cooking up new items for my shop, I'm sewing fabric baskets, taking care of our honeybees, pitching in on the latest project at my son's, or planning a trip somewhere with my daughter. Here, I'll share a "day in the life" at Tailored Touches! 
Sign up for the newsletter for an instant 10% off coupon.
Categories
All
Archives
February 2019
|





 RSS Feed
RSS Feed


